![]()
![]()
![]()
![]()
|
|
CELL DIFFERENTIATION AND DEVELOPMENT OF CELL MODELS |
MEMBERS AND COLLABORATORS
|
Name |
Category |
|
| Carmen Marín Vieira (Coord) | TU ULE | carmen.marin@unileon.es |
|
Inmaculada Diez Prieto |
TU ULE |
idiep@unileon.es |
|
María José Cano Rábano |
TU ULE |
maria.cano@unileon.es |
|
Margarita Marqués Martínez |
PCD ULE |
mmarm@unileon.es |
|
Alfonso Fernández Corona |
Jefe Sección Obs/GinecologíaCAULE |
alfonsofernandezcorona@yahoo.es |
|
Mª Elena Lorenzo Marcos |
Licen. Especial. Obs/Ginecología CAULE |
eleloma@yahoo.es |
| Marta Martín López | Becaria JCyL | marta.martin@unileon.es |
| Sandra Fuertes Alvarez | Becaria JCyL | sfuea@unileon.es |
OBJECTIVES
|
1. Generation and development of in vitro differentiation models from stem cells (mESC, ASC and iPSC) and its applications for drug screening. |
|
2. Development of in vitro angoigenic assays. |
| 3. Study of the molecular mechanisms that regulate stem cell pluripotency and differentiation. |
|
4. Study of the role of the p53 family members in stem cell biology. |
ACTIVITIES
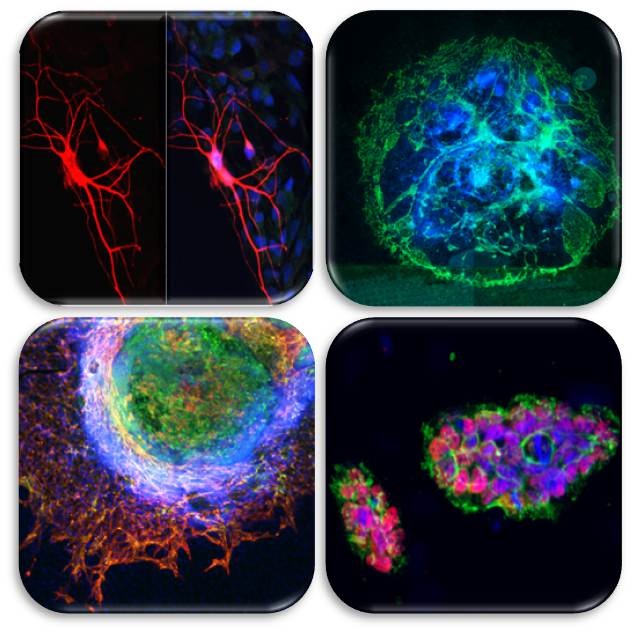
Our research focuses in investigating the role of p53 family members in stem cell biology and cell differentiation. For this purpose, we use mouse embryonic stem cells (mESC) as well as adult stem cells, with particular interest in neural stem cells. Recently, we have generated induced pluripotent stem cells (iPSC) from embryonic fibroblasts derived from genetically modified mice (p53KO y p73KO) (Martin-Lopez et al., in preparation).
The p53 gene family is constituted by the transcription factors p53, p73 and p63. Despite their sequence homology, the p53 family members possess both common as well as non-overlapping functions. There is evidence that suggest that p73 maintains some unique functions not shared with p53, like the p73-specific role in cellular differentiation and development. We have demonstrated that p73 function is necessary for neural differentiation mediated by Ras, showing for the first time that there is a signaling loop between Ras-dependent MAPK cascade activation and p73 function (Fernandez et al., 2006). We have also shown that p73 plays a critical role in erythroid differentiation (Marqués-García et al., 2009). Moreover, our recent work using 2D and 3D mESC differentiation models, demonstrates that p73 functional inhibition attenuates endothelial differentiation, impairs vascular morphogenesis and sprouting, pointing out p73 as an important player in the regulation of endothelial cell differentiation and angiogenesis (Fernandez-Alonso et al., in preparation).
Stem cells have triggered a revolutionary progress in analyzing gene function in vitro. Increasing evidence suggests that signaling pathways and properties associated with stem cells are important in the development of certain diseases. Therefore, investigation of the cellular and molecular mechanisms involved in stem cell biology (self-renewal, pluripotency, differentiation) could be very valuable to understand disease pathogenesis and, in some cases, to develop suitable treatments. In addition, the new advances which allow the restoration of cell pluripotency by the ectopic co-expression of reprogramming factors in somatic cells, have provided powerful opportunities for modelling human diseases and new possibilities for personalized regenerative cell therapies. In this regard, we have identified TP73 as a positive regulator of self-renewal of neural progenitor cells, with a role in the maintenance of the neurogenic capacity (Gonzalez-Cano et al., 2010). These results propose p73 as an important player in the development of neurodegenerative diseases and a potential therapeutic target. Furthermore, recent work in our laboratory has demonstrated the functional interaction between p73 and the neuronal fate determinant, TRIM32. Our data revealed the existence of a regulatory feed-back loop between TAp73 and TRIM32, similar to that of p53 and MDM2, with important implication in physiological processes, and probably in diseases like Parkinson or cancer (Gonzalez-Cano et al., 2013).
We are also interested in adapting the established cellular models for the screening of pharmacologically relevant compounds.
Financial support:
National Funding (Spanish Ministry of Science)
-
Functional interaction between p73 and p53 in the regulation of stem cells self-renewal and pluripotency, and in the process of cellular reprogramming. 2012-2015.
-
Determination of TP73 functions in the biology of hematopoietic and neural stem cells. 2009-2012.
-
Study of the cross-talk between Ras and p73: consequences in the processes of senescence, differentiation and chemosensitivity. 2006-2009.
-
P73 function in processes of neuronal differentiation anad apoptosis. “Cross-talk” with cell cycle regulators E2F and Myc. 2002-2005
Regional Funding
-
Study of the coordinated functions of TP53 and TP73 tumor suppresors in the biology of neural stem cells. Relevance to aging and neurodegenerative diseases. 2010-2012.
-
Analysis of the functional inhibition of p53 family members in mouse embryonic stem cells: effect on differentiation and response to genotoxic damage. 2007-2010
-
Study of the role of p53 family members on the maintenance of genomic stability and differentiation capacity of murine embryonic stem cells. 2006-2008.
-
Study of bimodal function of p73 isoforms in myeloid leukemia. 2005-2006.
Participation in R&D programs with companies
-
Innovative solutions to accelerate the identification and development of new drugs for the treatment of diseases of the nervous system. 2011-2013.
-
Development and validation of new screening procedures for the identification of candidates to pharmaceuticals from a natural product collection. 2007-2008.
-
Study of the pharmacological properties of new antitumoral compounds of sea origin. 2005-2006.
-
Study of DNp73 function in chemosensitivity using mouse cancer models. 2005.
TECHNOLOGICAL OFFER
|
1. Development of cellular model systems to study cell differentiation and to utilize them for the screening of pharmacologically relevant compounds |
SELECTED PUBLICATIONS
-
Gonzalez–Cano, L.; Hillje, A.L., Fuertes-Alvarez, S., Marques, M.M, Blanch, A., Ian, R.W., Irwin, M.S., Schwamborn, J. & Marin, M.C. (2013). Regulatory feedback loop between TP73 and TRIM32. Cell Death and Disease, 4, e704.
-
Gonzalez-Cano.L., Herreros-Villanueva, M., Fernandez-Alonso, R., Ayuso-Sacido, A., Garcia-Verdugo, J.M., Silva, A., Marques, M.M. & Marin, M.C. (2010). p73 deficiency results in impaired self-renewal and premature neuronal differentiation of mouse neural progenitors independently of p53. Cell Death and Disease, 1, e109.
-
Marques-García, F., Ferrandiz, N., Fernandez-Alonso, R., Gonzalez-Cano, L., Herreros-Villanueva, M., Rosa-Garrido, M., Fernandez-Garcia, B., Vaque, J.P., Marques, M.M., Leon, J. & Marin, M.C. (2009). p73 plays a role in erythroid differentiation. Journal of Biological Chemistry, 248, 21139 - 21156.
-
Fernandez-Garcia, B., Vaque, J.P., Herreros-Villanueva, M., Marques-García, F., Castrillo, F., Fernandez-Medarde, A., Leon, J. & Marín, M.C. (2007). p73 cooperates with Ras in the activation of MAP kinase signaling cascade. Cell Death Differentiation, 14: 254-265.
-
Fernandez-Alonso. R., Martin-Lopez, M., Gonzalez-Cano L., Garcia- S., Diaz-Prieto I., Claesson-Welsh L., Marques, M.M. & Marin, M.C. TP73 is required for endothelial cell differentiation, migration and the formation of vascular networks (submitted) .
-
Martin-Lopez, M., Marques, M.M., Navarro-Montero, O., Fuertes-Alvarez, S., Balboa-Alonso, D., Weltner, J., Menendez-Bujan, P. & Marin, M.C. Effect of p73 deficiency on somatic cell reprogramming (in preparation) .
CONTACT
![]()
![]()
![]()
![]()
![]()
![]()

![]()

![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()